Research
We are interested in the fundamental mechanisms that direct human brain development. This process involves the creation of billions of cells which must differentiate into the specific cells type, migrate to their appropriate destinations, and establish proper connections with other cells — all on a strict timeline.
Genetic mutations can disrupt or alter the steps of brain development. Atypical cell growth or accumulation of cells in abnormal locations in the brain, and/or defects in cell function may lead to abnormal brain structure or function.
After development, the brain continues to be a highly plastic and dynamic organ responsible for human consciousness and behavior. The Walsh lab studies how this remarkable and complex process unfolds, and what happens when things go wrong. We approach this question from four interrelated perspectives: Evolution, Neurological Disorders, Cell Lineage and Aging, and Functional Analysis and Modeling.
Chris Walsh established his own laboratory in 1993. Immersed in the complex beauty of human genetics and the potential to identify genes and mechanisms that control the stem cells and neurons that build the human cerebral cortex, his lab has identified more than three dozen neurological disease genes.
Mutations in these genes result in a range of neurological and cognitive disorders, including: epilepsy, intellectual disability, motor deficits, and autism spectrum disorders (ASD).
Fundamental to these discoveries has been the generous collaboration of families, physicians and scientists around the world. Our study of autosomal recessive disorders especially benefitted from colleagues in Israel, Kuwait, Saudi Arabia, Oman, the UAE, and other countries of the Middle East, where marriage between cousins is common. These consanguineous families were critical for revealing that ASD can be caused by recessive mutations that disrupt protein structure, as well as mutations that can disrupt “noncoding” parts of the genome that affect patterns or levels of gene expression rather than the structure of proteins.
A serendipitous finding was that some of the genes we identified as essential for human brain development bear genomic hallmarks identifying them as targets of evolutionary selection on the human brain. Systematic changes in these genes are evident in species leading to humans. This finding led to the initiation of the Allen Discovery Center for Human Brain Evolution. More about our brain evolution work appears below.
More recent work has contributed to the understanding of somatic mosaic mutations—present in some of a child’s cells, but not all of them—as the most common cause of intractable focal epilepsy that requires brain surgery to treat, and as another important cause of ASD. These somatic mosaic mutations turn out to be amazingly common—with 2-3 occurring every time a cell divides. We are using them to make a map of the cell lineage of the human brain using postmortem specimens to understand how cell lineage contributes to the neuronal diversity and structural patterning of the human brain.
Finally, we have developed methods to analyze the genome of a single neuron. We have found that an individual neuron differs by hundreds to thousands of mutations compared to the next neuron, reflecting developmental mutations, but also mutations that occur in a single, nondividing neuron as it ages. We call this phenomenon “geno-senium” (genome aging), and are studying its relevance to normal brain aging, and age-related degenerative disorders of the human nervous system. Read more about this under Cell Lineage and Aging, below.
Walsh Lab Gene Discoveries
Genes that regulate neural stem cell proliferation
AKT3, ARFGEF2, ATP1A3, PNKP, NDE1, ZNF335, JAM3, WDR62, CHMP1A, KATNB1, DONSON, SCN3A, EXOC7, EXOC8.
Genes that regulate neuronal migration
DCX, RELN, FLNA, GTDC2, GPR56, CEP85.
Gene that determines neuronal survival
QARS.
Genes involved in neuron differentiation and function
PAK3, METTL23, EMC10, DCC, AHI1, and TRAPPC9.
Career Highlights
2002. Named Investigator, Howard Hughes Medical Institute.
2003-2007. Director, Harvard-MIT combined MD-PhD training program.
2005. Accepts position as Chief, Division of Genetics (now Genetics and Genomics), Boston Children’s Hospital, dedicated to research and treatment to help children with rare genetic disorders.
2017. Allen Discovery Center for Human Brain Evolution is established at Boston Children’s Hospital and Harvard Medical School.
2021. Awarded Gruber Neuroscience Prize.
Autism Genetics and Human Brain Evolution
Christopher A. Walsh, MD, PhD; May 2021
This one-hour public outreach lecture, followed by a very worthwhile Q&A exchange among Harvard scientists, was sponsored by the Hock E. Tan and K. Lisa Yang Center for Autism Research at Harvard University.
Brain Evolution
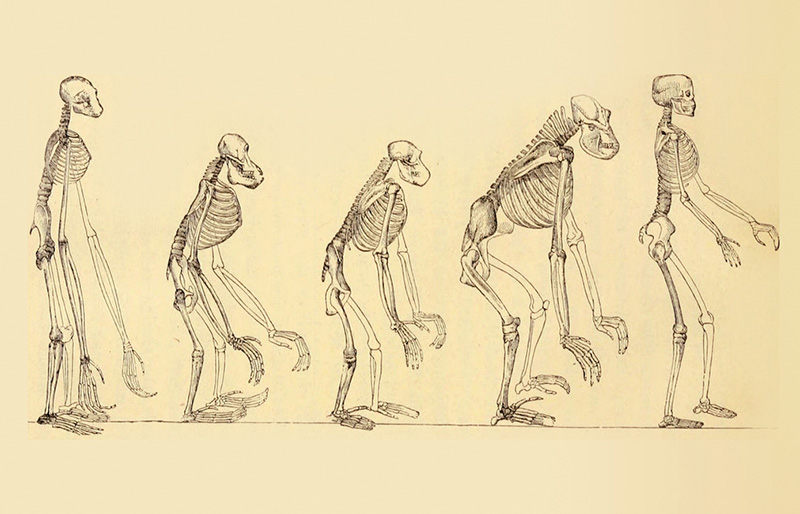
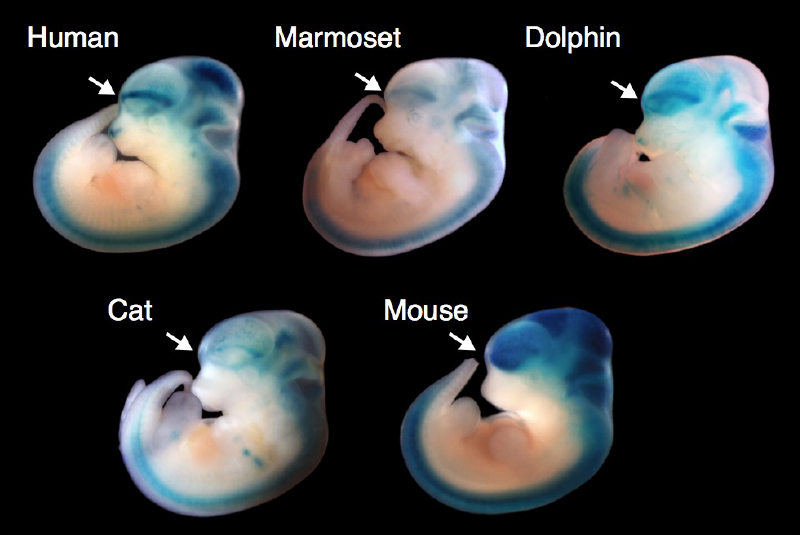
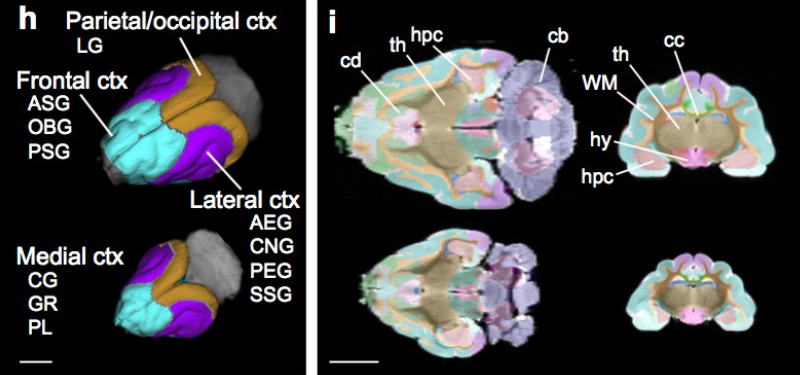
What changes in the brain brought about human culture as we know it, and when those changes occurred is a question being asked by the Paul G. Allen Discovery Center for Human Brain Evolution at Boston Children’s Hospital and Harvard Medical School. The Walsh lab, together with the Reich and Greenberg labs, are studying DNA from ancient humans, dynamic molecular responses of human and non-human primate neurons to stimuli, and genetic mutations that disrupt higher lever social functions in humans, to address this question. Gaining a better understanding of how the human brain evolved is not just an extremely interesting question in its own right, but will also provide us with a better understanding of how to treat diseases like autism and schizophrenia, and an appreciation of how well model organisms can represent human neurological disorders.
Visit the website of the Allen Discovery Center for Brain Evolution here. >
Neurological Disorders: Translational Medicine
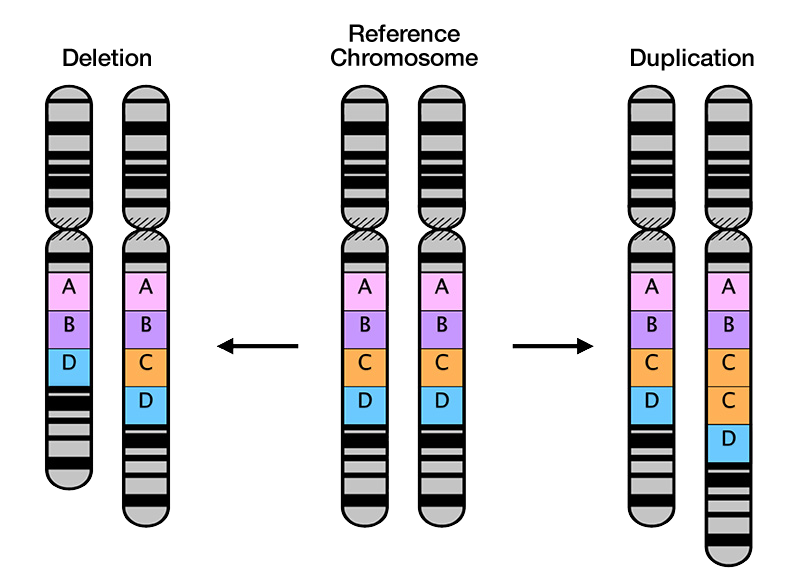
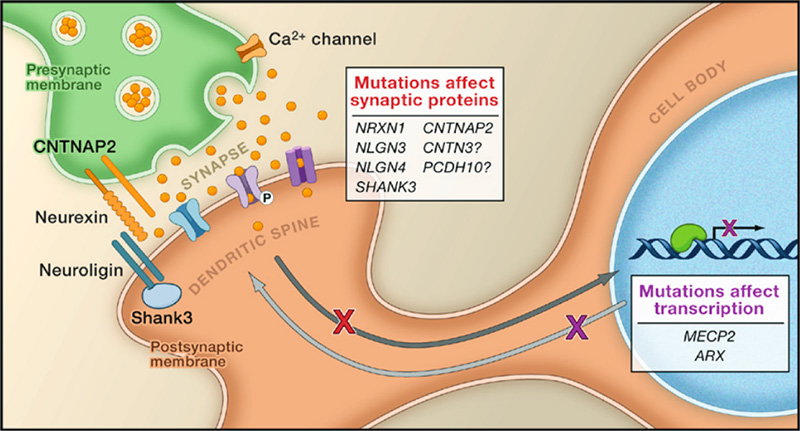
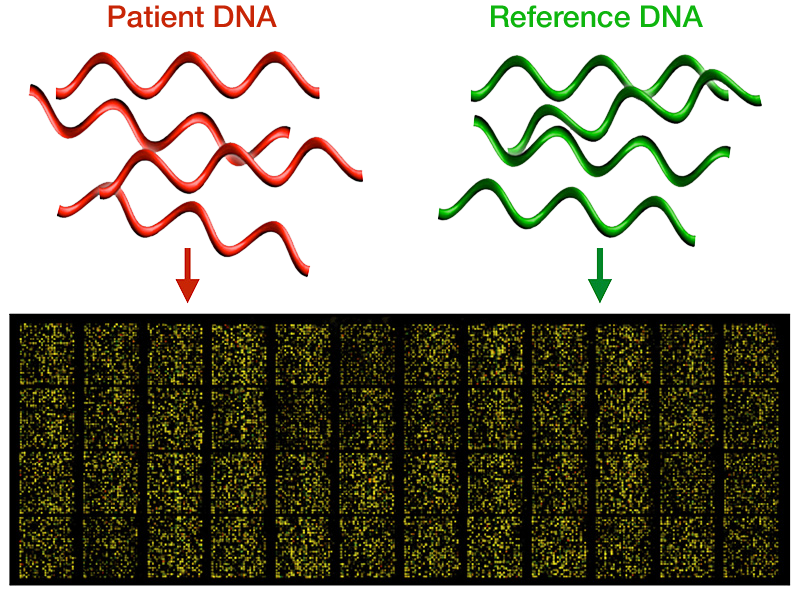
In any complex system there are many things that can and do go wrong, and the brain is no exception. The Walsh Lab is interested in discovering the genetic causes of these disorders. Working with clinicians and families afflicted with neurological diseases around the world, we have identified genes associated with dozens of disorders.
To expand our reach, we are actively involved with the Center for Mendelian Genomics, the Autism Sequencing Consortium, the Centers for Common Disease Genomics (CCDG), and GeneMatcher. Linking disorders to specific genetic mutations allows for clinical testing and is the first step in developing treatments and cures.
Read about the work of our clinics here. >

Cell Lineage and Aging
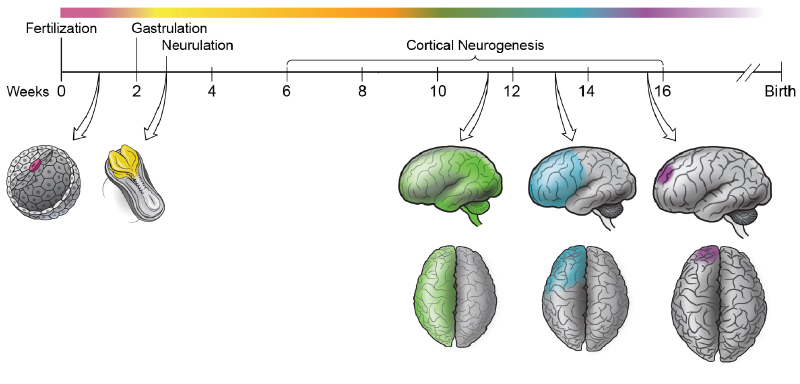
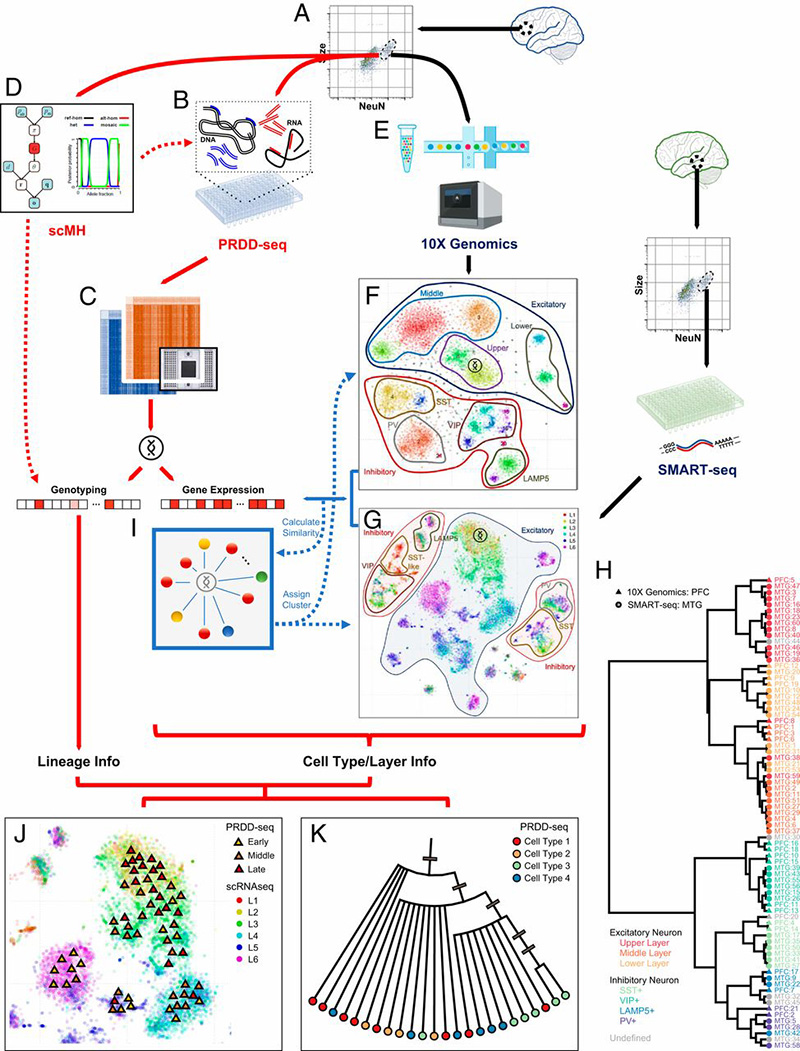
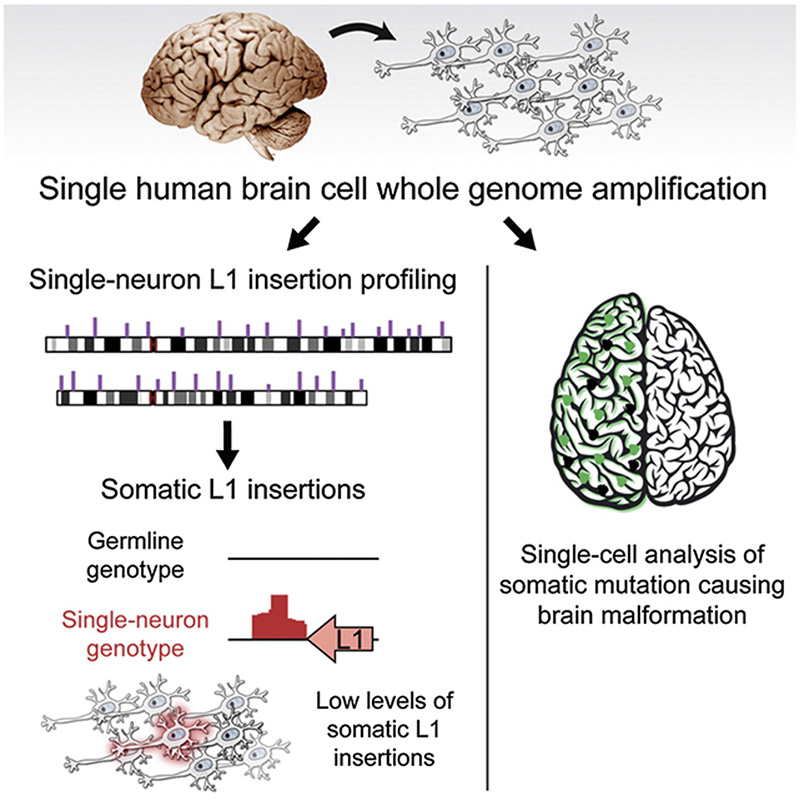
The Walsh lab was one of the first labs to develop and use single cell genome sequencing. This technology has allowed us to address several important questions about brain development and aging. What happens to the DNA of individual neurons (cells which do not divide and are mostly never replaced) as we age? How many embryonic stem cells are needed to form the brain? How disperse is the migration pattern of neurons during brain development, and does this dispersion pattern correlate with physical brain structure? Addressing these questions will provide valuable insight into how the brain forms and ages, and help us better understand neurological disorders accolated with aging and incorrect migration of neurons.

Functional Analysis and Modeling
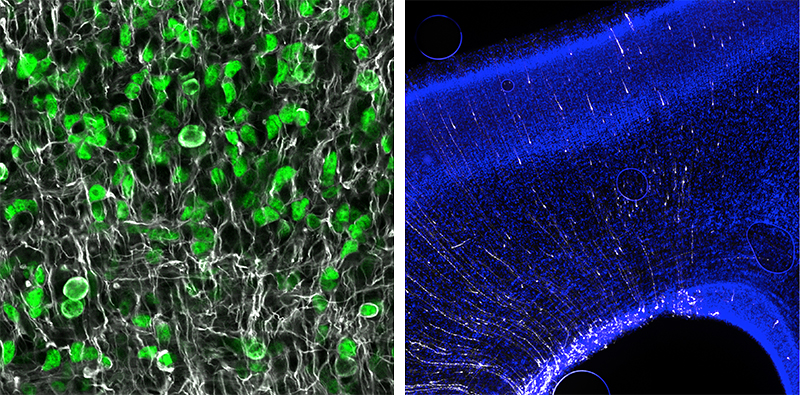
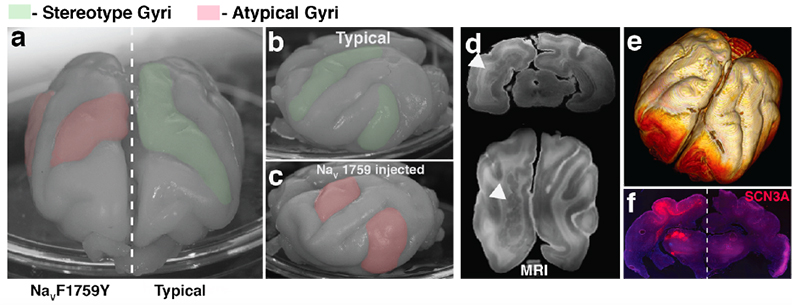

Identifying genes and functional regions that result in neurological disorders when mutated (either germline or somatic) is integral and important in helping to detect and develop therapies for these disorders. It also provides a window into how the brain develops. How do these mutations cause the observed brain phenotypes? We use human cell lines, organoids, and animal models (zebrafish, mice, and ferrets) in conjunction with bioinformatic tools to investigate impact these mutations at the molecular, cellular, and organism level. These investigations also provide a further step towards understanding the disease and possible treatment by helping to identify which molecular pathways are being disrupted, and by identify potential drug targets.